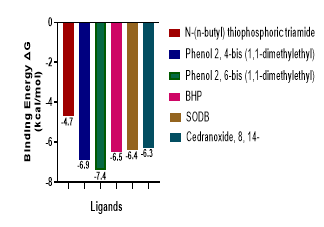Archachatina marginata Slime Bioactive Compounds Combat Peptic Ulcer via Inhibition of H+/K+-ATPase and Helicobacter pylori Urease: A Computational Study
Main Article Content
Abstract
The search for natural treatment of gastric ulcers continues to attract the attention of researchers, due to the side effects of the existing synthetic antiulcer agents. The aim of this study was to examine the in silico inhibitory effects of Archachatina marginata slime on Helicobacter pylori urease and gastric H+/K+-ATPase, which are implicated in the pathophysiology of gastric ulcer. We identified the bioactive constituents of pulverized A. marginata slime using gas chromatography–mass spectrometry (GC –MS), and the identified ligands were docked using PyRx and BIOVIA Discovery Studio. The pharmacokinetics (ADME) and physicochemical properties of hit ligands were predicted using SwissADME, and their toxicity was assessed using pkCSM. The extracted snail slime, upon identification by GC-MS, showed twenty-three (23) peaks, corresponding to forty-three (43) compounds. Phenol, 2,6-bis(1,1-dimethylethyl)- (P26BD), benzaldehyde, 3,5-dimethyl- (BAD), 4-butyl-5-(3-methylbutyl)-6-(1-methylethenyl)-2H-pyran-2-one (BHP), spiro [2.5] octane, 3,3-dimethyl-2-(1-buten-3-on-1-yl)- (SODB), and cedranoxide, 8,14- (CO) had higher binding affinity against urease (-7.4, -5.7, -6.5, -6.4 and -6.3 kcal/mol, respectively) than the standard inhibitor, N-(n-butyl) thiophosphoric triamide (NBPT) (-4.7 kcal/mol). Of all the forty-three (43) ligands, phenol, 2,4-bis(1,1-dimethylethyl)- (P24BD) with -7.4 kcal/mol) showed a predicted binding affinity close to that of the control (Omeprazole with -7.8 kcal/mol binding affinity). Phenol, 2,6-bis(1,1-dimethylethyl)- (P26BD) showed a higher binding affinity against H+/K+-ATPase (-8.0 kcal/mol) than the omeprazole (-7.89 kcal/mol), a potent proton pump inhibitor. Thus, these compounds may be potent in the treatment of gastric ulcers, since they have demonstrated strong docking affinities against the proteins implicated in gastric ulceration.
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Sverdén E, Lars A, Jason M, Dunn M, Jesper L. Peptic ulcer disease. Brit Med J. 2019; 367 (3): 1-8. https://10.1136/bmj.l5495
Majumdar D, Looi S. Helicobacter pylori infection and peptic ulcers. Medicine (Abingdon). 2024; 52(3):152–160.
https://doi.org/10.1016/j.mpmed.2023.12.006
Collatuzzo G, Dajti E, Secco M, Bazzoli F, Boffetta P, Zagari RM. Burden of gastric cancer attributable to Helicobacter pylori in 27 countries from seven geographic regions in 2022. Gastric Cancer. 2025; 28(1):1–11.
https://doi.org/10.1007/s10120-025-01677-9
Yibirin M, De Oliveira D, Valera R, Plitt AE, Lutgen S. Adverse effects associated with proton pump inhibitor use. Cureus. 2021; 13(1): 1-6 https://doi.org/10.7759/cureus.12759
Ghouri YA, Aslam S, Jafri W. Helicobacter pylori infection. Cleve Clin J Med. 2021; 88(6):347-354. https://doi.org/10.3949/ccjm.88a.20193
Rani L, Ramakrishnan LM, Vinodhin D, Krishnaaveni GP, Bhuvaneshwari B, Sudarvizhi M, Reena J, Priyanka Ramya T. Molecular docking study of lead compounds from Elathy Chooranam a Siddha formulation against Helicobacter pylori urease. J Pharm Negat Results. 2023; 14(3):1470-1477.
Jaiswal F, Rai AK, Wal P, Wal A, Singh SP. Peptic ulcer: a review on etiology, pathogenesis and treatment. Asian J Pharm Educ Res. 2021; 10(4):1-17.
Merkhan M, Mustafa Y, Hassan E. Safety and efficacy of proton-pump inhibitors are relevant to their distinctive chemical structures and physicochemical properties. Int J Pharm Stud. 2022; 6(2):1–11.
Gheorghe C, Pascu O, Iacob R. Adverse effects associated with proton pump inhibitor therapy. Maedica (Bucur). 2012; 7(4):309-314.
Nwodo NJ, Okota JM, Ezugwu CO, Attama AA. Anti-ulcer potentials of phylum mollusca (tropical snail) slime. Asian Pac J Trop Med. 2009; 2(3):23-28.
Ibrahim M, Mohammed H, Warra Z, Abdulrahman A, Sabo Y, Ibrahim I, Jajere GU, Victor A, Oyi R. Physicochemical and genetic diversity studies of Vitellaria paradoxa in Northern Nigeria. J Curr Biomed Res. 2022; 2(1):19–37.
https://doi.org/10.54117/jcbr.v2i1.4
Rahman N, Khalil N, Khan S, Almutairi MH, Almutairi BO, Alam M. Multispectroscopic and molecular docking studies on the interaction of diltiazem hydrochloride with bovine serum albumin and its application to the quantitative determination of diltiazem hydrochloride. J King Saud Univ Sci. 2021; 34 (4): 1-8.
Kuipers EJ, Thijs JC, Festen HPM. The pathogenesis of peptic ulcer disease. N Engl J Med. 2021; 384(25):2406–2416. https://doi.org/10.1056/NEJMra2028892
Li B, Lan X, Wang L, Zhao J, Ding J, Ding H, Lei J, Wei Y, Zhang W. Proton-pump inhibitor and amoxicillin-based triple therapy containing clarithromycin versus metronidazole for Helicobacter pylori: a meta-analysis. Microb Pathog. 2020; 142 (1):1-8.
https://doi.org/10.1016/j.micpath.2020.104075
Kamada T, Satoh K, Itoh T, Ito M, Iwamoto J, Okimoto T, Kanno T, Sugimoto M, Chiba T, Nomura S, Mieda M, Hiraishi H, Yoshino J, Takagi A, Watanabe S, Koike K. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol. 2021; 56(5):459–473.
https://doi.org/10.1007/s00535-021-01769-0
Amah AK, Ewa O, Karimah MR, Elendu MU, Yunusa Z. Effect of Archachatina marginata mucin on the aggressive factors of gastric ulcer challenged Wistar rat stomach tissue. Biol Pharm Sci. 2019; 9(1):77–82.
Mehrotra R, Agarwal S. An overview of molecular docking. JSM Chem. 2016; 4(2):1024.
Guedes IA, Magalhães CS, Dardenne LE. Receptor–ligand molecular docking. Biophys. Rev. 2014; 6 (6): 75-87.
Du X, Li Y, Xia YL. Insights into protein-ligand interactions: mechanisms, models, and methods. Int J Mol Sci. 2016; 17(2):1-34.
Kawade D, Nikhil S, Payal B, Payal H, Nidhi D. Peptic ulcer: a review on its etiology, pathogenesis and pharmacotherapy. World J Pharm Res. 2020; 9(5):558-583.
Khan A, Abbasi SW, Shah FA, Rasheed F, Qazi NG, Pervez S, Khalil AAK, Ali F, Aziz N, Seo SY. Ethnopharmacological basis of Rumex hastatus D. Don in gastrointestinal diseases with focusing effects on H⁺/K⁺-ATPase, calcium channels, and PDE inhibition. Molecules. 2022; 27(18):5919. https://doi.org/10.3390/molecules27185919
Gonfa YH, Beshah F, Tadesse MG, Bachheti A, Bachheti RK. Phytochemical investigation and potential pharmacologically active compounds of Rumex nepalensis: an appraisal. Beni-Suef Univ J Basic Appl Sci. 2021; 10(18):1–11.
Panigrahi N, Pasha A, Mondal S. Synthesis of novel aryl (4-aryl-1H-pyrrol-3-yl) (thiophen-2-yl) methanone derivatives: molecular modelling, in silico ADMET, and anti-ulcer activities. Anti-Inflamm Anti-Allergy Agents Med Chem. 2021; 20(2):135–147.
https://doi.org/10.2174/1871520620666201120161327
Laloo D, Sinha SK, Prasad SK, Hemalatha S. Gastric H+/K+-ATPase inhibitory effects of the active constituent isolated from Potentilla fulgens roots: An in vivo and in silico molecular docking studies. Phytomedicine Plus. 2021; 1(1):1–10.
Han Y, Zhang J, Hu CQ, Zhang X, Ma B, Zhang P. In silico ADME and toxicity prediction of ceftazidime and its impurities. Front Pharmacol. 2019; 10:434.
Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 1997; 29 (2):413-580.
Ohno Y, Hisaka A, Suzuki H. General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs. Clin Pharmacokinet. 2007; 46 (8):681-696.
Busti AJ, Herrington JD, Daves BJ, McKeever GC. What is the process to determine if medications are to be classified as weak, moderate or strong inhibitors of CYP3A4? PW Drug Interact Newsl. 2009; 1(39):1-3.
Hebert MF. Impact of pregnancy on maternal pharmacokinetics of medications. Clin Pharmacol Pregnancy. 2013; 20(3):17-39.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012; 2 (1): 1-10. https://doi.org/10.5402/2012/195727
Jia C, Li J, Hao G, Yang G. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov Today. 2020; 25(1):248-258. https://doi.org/10.1016/j.drudis.2019.10.014
Meanwell NA. Improving drug candidates by design: a focus on physicochemical properties as a means of improving compound disposition and safety. Chem Res Toxicol. 2011; 24(6):1420–1456.
Alabi MA, Abiola MM, Yakub NT, Ajani EO. In vitro anti-inflammatory and anti-ulcerogenic potential of ethanol extract of Carica papaya leaves: phytochemical and molecular docking studies. Trop J Phytochem Pharm Sci. 2025; 4(8):341–354. http://www.doi.org/10.26538/tjpps/v4i8.1/5