In Vitro Anti-Inflammatory and Anti-Ulcerogenic Potential of Ethanol Extract of Carica papaya Leaves: Phytochemical and Molecular Docking Studies
Main Article Content
Abstract
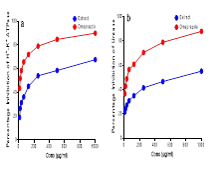
Pharmaceutical treatments for inflammation and ulcers often have side effects, prompting interest in plant-based alternatives. This study investigates the anti-inflammatory and anti-ulcerogenic potential of ethanol extract of Carica papaya leaves using in vitro assays and molecular docking. The leaves were subjected to ethanol extraction, after which the resulting extract underwent GC-MS analysis and in vitro assays. The phytochemicals identified from the GC-MS profile were subsequently analyzed through molecular docking studies for further evaluation. The extract demonstrated significant anti-inflammatory activity by inhibiting protein denaturation (IC₅₀: 180.70±2.25 µg/ml), membrane stabilization (IC₅₀: 192.60±2.29 µg/ml), and proteinase activity (IC₅₀: 1289.00±3.11 µg/ml). Its anti-ulcerogenic potential was evident in its inhibitory effects on H⁺/K⁺-ATPase (IC₅₀: 196.10±2.29 µg/ml) and urease (IC₅₀: 700.90±2.85 µg/ml), suggesting a role in gastric protection. Gas Chromatography-Mass Spectrometry (GC-MS) analysis identified biologically active compounds, such as phenolic acids and long-chain fatty acid esters, known for their anti-inflammatory and gastroprotective properties. Molecular docking analyses additionally confirmed the therapeutic potential of 2,3-Benzofurandione 2-monooxime, 3-(4-Nitrophenyl) propiolic acid, and Phthalic acid, di-(1-hexen-5-yl) by demonstrating strong interactions with cyclooxygenase-2 (COX-2) and H⁺/K⁺-ATPase enzymes, with binding affinities comparable to standard drugs like celecoxib and omeprazole respectively. These results offer scientific validation supporting the traditional use of C. papaya leaves in managing inflammatory and ulcerative conditions. Further in vivo studies and clinical trials are necessary to confirm their efficacy and safety for therapeutic applications.
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Lin D, Jin Y, Shao X, Xu Y, Ma G, Jiang Y, Hu D. Global, regional, and national burden of inflammatory bowel disease, 1990-2021: Insights from the global burden of disease 2021. Int J Colorectal Dis. 2024;39(1):139. Doi: 10.1007/s00384-024-04711-x
M'Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33-47. Doi: 10.4137/CGast.S12731
Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai, N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822-1832. Doi: 10.1038/s41591-019-0675-0
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204-7218. Doi: 10.18632/oncotarget.23208
Moore RA, Derry S, Phillips CJ, McQuay HJ. Nonsteroidal anti-inflammatory drugs (NSAIDs), cyxlooxygenase-2 selective inhibitors (coxibs) and gastrointestinal harm: review of clinical trials and clinical practice. BMC Musculoskelet Disord. 2006;7:79. Doi: 10.1186/1471-2474-7-79
Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018;9(1):143-150. Doi: 10.14336/ad.2017.0306
Ansari P, Reberio AD, Ansari NJ, Kumar S, Khan JT, Chowdhury S, Abd El-Mordy FM, Hannan JMA, Flatt PR, Abdel-Wahab YHA, Seidel V. Therapeutic Potential of Medicinal Plants and Their Phytoconstituents in Diabetes, Cancer, Infections, Cardiovascular Diseases, Inflammation and Gastrointestinal Disorders. Biomedicines. 2025;13(2). Doi: 10.3390/biomedicines13020454
Hao DC, Xiao PG. Genomics and Evolution in Traditional Medicinal Plants: Road to a Healthier Life. Evol Bioinform Online. 2015;11:197-212. Doi: 10.4137/ebo.S31326
Petrovska BB. Historical review of medicinal plants' usage. Pharmacogn Rev. 2012;6(11):1-5. Doi: 10.4103/0973-7847.95849
Koul B, Pudhuvai B, Sharma C, Kumar A, Sharma V, Yadav D, Jin J-O. Carica papaya L.: A Tropical Fruit with Benefits beyond the Tropics. Diversity. 2022; 14(8).
Singh SK, Thakur K, Sharma V, Saini M, Sharma D, Vishwas S, Kakoty V, Pal RS, Chaitanya MVNL, Babu MR, Gupta S, Rehman Zu, Smriti Singla M, Gupta G, Jakhmola V, Pinto TdJA, Kumbhar P, Disouza J, ... Gadewar MM. Exploring the multifaceted potential of chlorogenic acid: Journey from nutraceutical to nanomedicine. S Afri J Bot. 2023;159:658-77. Doi: Doi: 10.1016/j.sajb.2023.06.038
Arora R, Malhotra P, Sharma A, Haniadka R, Yashawanth HS, Baliga MS. Chapter 45 - Medicinal Efficacy of Indian Herbal Remedies for the Treatment of Arthritis. In: Watson RR, Preedy VR, editors. Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases. San Diego: Academic Press; 2013. p. 601-17. Doi: 10.1016/B978-0-12-397156-2.00250-7
Ugbogu EA, Dike ED, Uche ME, Etumnu LR, Okoro BC, Ugbogu OC, Adurosakin OE, Chinma CE, Ohaeri E, Iweala EJ. Ethnomedicinal uses, nutritional composition, phytochemistry and potential health benefits of Carica papaya. Pharmacol Res - Mod Chin Med. 2023;7:100266. Doi: 10.1016/j.prmcm.2023.100266
Sharma A, Sharma R, Sharma M, Kumar M, Barbhai MD, Lorenzo JM, Sharma S, Samota MK, Atanassova M, Caruso G, Naushad M, Radha Chandran D, Prakash P, Hasan M, Rais N, Dey A, Mahato DK, Dhumal S, ... Mekhemar M. Carica papaya L. Leaves: Deciphering Its Antioxidant Bioactives, Biological Activities, Innovative Products, and Safety Aspects. Oxid Med Cell Longev. 2022;2022:2451733. Doi: 10.1155/2022/2451733
Singh SP, Kumar S, Mathan SV, Tomar MS, Singh RK, Verma PK, Kumar A, Kumar S, Singh RP, Acharya A. Therapeutic application of Carica papaya leaf extract in the management of human diseases. Daru. 2020;28(2):735-44. Doi: 10.1007/s40199-020-00348-7
Chaijan S, Chaijan M, Uawisetwathana U, Panya A, Phonsatta N, Shetty K, Panpipat W. Phenolic and Metabolic Profiles, Antioxidant Activities, Glycemic Control, and Anti-Inflammatory Activity of Three Thai Papaya Cultivar Leaves. Foods. 2024; 13(11).
Kumar A, P N, Kumar M, Jose A, Tomer V, Oz E, Proestos C, Zeng M, Elobeid T, K S, Oz F. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules. 2023;28(2). Doi: 10.3390/molecules28020887
Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Current Opinion in Food Science. 2016;8:33-42. Doi: 10.1016/j.cofs.2016.02.002
Chagas M, Behrens MD, Moragas-Tellis CJ, Penedo GXM, Silva AR, Gonçalves-de-Albuquerque CF. Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxid Med Cell Longev. 2022;2022:9966750. Doi: 10.1155/2022/9966750
Kong YR, Jong YX, Balakrishnan M, Bok ZK, Weng JKK, Tay KC, et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology (Basel). 2021;10(4).
Koo I, Kim S, Zhang X. Comparative analysis of mass spectral matching-based compound identification in gas chromatography-mass spectrometry. J Chromatogr A. 2013;1298:132-138. Doi: 10.1016/j.chroma.2013.05.021
Osman NI, Sidik NJ, Awal A, Adam NA, Rezali NI. In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J Intercult Ethnopharmacol. 2016;5(4):343-9. Doi: 10.5455/jice.20160731025522
Ameena A, Meignana AA, Karthikeyan R, Rajeshkumar S. Evaluation of the Anti-inflammatory, Antimicrobial, Antioxidant, and Cytotoxic Effects of Chitosan Thiocolchicoside-Lauric Acid Nanogel. Cureus. 2023;15(9):e46003. Doi: 10.3389/pore.2021.1610136
Anosike CA, Obidoa O, Ezeanyika LU. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). Daru. 2012;20(1):76. Doi: 10.1186/2008-2231-20-76
Yadav P, Ganeshpurkar A, Rai G. In vitro H(+) -K(+) ATPase inhibitory potential of methanolic extract of Cissus quadrangularis Linn. Pharmacognosy Res. 2012;4(2):123-6. Doi: 10.4103/0974-8490.94738
Fiske CH, Subbarow Y. The Colorimetric Determination of Phosphorus. J Biol Chem. 1925;66(2):375-400. Doi: 10.1016/S0021-9258(18)84756-1
Kumar M, Sikri N, Chahal S, Sharma J, Sharma B, Yadav P, Bhardwaj M, Vashishth D, Kadyan P, Kataria SK, Dalal S. Urease Inhibitory Kinetic Studies of Various Extracts and Pure Compounds from Cinnamomum Genus. Molecules. 2021;26(13). Doi: 10.3390/molecules26133803
Gonfa YH, Tessema FB, Bachheti A, Rai N, Tadesse MG, Nasser Singab A, Chaubey KK, Bachheti RK. Anti-inflammatory activity of phytochemicals from medicinal plants and their nanoparticles: A review. Curr Res Biotechnol. 2023;6:100152. Doi: 10.1016/j.crbiot.2023.100152
Kim S, Thiessen PA, Bolton EE, Chen J, Fu G, Gindulyte A, Han L, He J.,He S, Shoemaker BA, Wang J, Yu B, Zhang J, Bryant SH. PubChem Substance and Compound databases. Nucleic Acids Res. 2016;44(D1):D1202-13. Doi: 10.1093/nar/gkv951
Kim S. Exploring Chemical Information in PubChem. Curr Protoc. 2021;1(8):e217. Doi: 10.1002/cpz1.217
Shelley JC, Cholleti A, Frye LL, Greenwood JR, Timlin MR, Uchimaya M. Epik: a software program for pKa prediction and protonation state generation for drug-like molecules. J Comput-Aided Mol Des. 2007;21(12):681-91. Doi: 10.1007/s10822-007-9133-z
Shamsian S, Sokouti B, Dastmalchi S. Benchmarking different docking protocols for predicting the binding poses of ligands complexed with cyclooxygenase enzymes and screening chemical libraries. Bioimpacts. 2024;14(2):29955. Doi: 10.34172/bi.2023.29955
Genheden S, Ryde U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov. 2015;10(5):449-61. Doi: 10.1517/17460441.2015.1032936
Dong L, Qu X, Zhao Y, Wang B. Prediction of Binding Free Energy of Protein-Ligand Complexes with a Hybrid Molecular Mechanics/Generalized Born Surface Area and Machine Learning Method. ACS Omega. 2021;6(48):32938-47. Doi: 10.1021/acsomega.1c04996
Zhou Z, Felts AK, Friesner RA, Levy RM. Comparative performance of several flexible docking programs and scoring functions: enrichment studies for a diverse set of pharmaceutically relevant targets. J Chem Inf Model. 2007;47(4):1599-608. Doi: 10.1021/acsomega.1c04996
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49(21):6177-96. Doi: 10.1021/jm051256o
Kiss AL. Inflammation in Focus: The Beginning and the End. Pathol Oncol Res. 2021;27:1610136. Doi: 10.3389/pore.2021.1610136
Kumarasinghe HS, Kim J-H, Kim S-L, Kim KC, Perera RMTD, Kim S-C, Kim SC, Lee DS. Bioactive constituents from Carica papaya fruit: implications for drug discovery and pharmacological applications. Appl Biol Chem. 2024;67(1):103. Doi: 10.1186/s13765-024-00962-y
Mutha RE, Tatiya AU, Surana SJ. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Futur J Pharm Sci. 2021;7(1):25. Doi: 10.1186/s43094-020-00161-8
Ranasinghe P, Ranasinghe P, Abeysekera WP, Premakumara GA, Perera YS, Gurugama P, Gunatilake SB. In vitro erythrocyte membrane stabilization properties of Carica papaya L. leaf extracts. Pharmacognosy Res. 2012;4(4):196-202. Doi: 10.4103/0974-8490.102261
Banik S, Biswas S, Karmakar S. Extraction, purification, and activity of protease from the leaves of Moringa oleifera. F1000Res. 2018;7:1151. Doi: 10.12688/f1000research.15642.1
Craik CS, Page MJ, Madison EL. Proteases as therapeutics. Biochem J. 2011;435(1):1-16. Doi: 10.1042/bj20100965
Drini M. Peptic ulcer disease and non-steroidal anti-inflammatory drugs. Aust Prescr. 2017;40(3):91-3. Doi: 10.18773/austprescr.2017.037
El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. 2018;14(4):447-60. Doi: 10.1080/17425255.2018.1461835
Sachs G, Shin JM, Hunt R. Novel approaches to inhibition of gastric acid secretion. Curr Gastroenterol Rep. 2010;12(6):437-47. Doi: 10.1007/s11894-010-0149-5
Shin JM, Munson K, Vagin O, Sachs G. The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch. 2009;457(3):609-22. Doi: 10.1007/s00424-008-0495-4
Hussain S, Guruvayoorappan C, Komal KP, Ennaganti S. Molecular docking analysis of doronine derivatives with human COX-2. Bioinformation. 2020;16(6):483-92. Doi: 10.6026/97320630016483
Oniga SD, Pacureanu L, Stoica CI, Palage MD, Crăciun A, Rusu LR, Crisan EL, Araniciu C. COX Inhibition Profile and Molecular Docking Studies of Some 2-(Trimethoxyphenyl)-Thiazoles. Molecules. 2017;22(9). Doi: 10.3390/molecules22091507
Sohilait MR, Pranowo HD, Haryadi W. Molecular docking analysis of curcumin analogues with COX-2. Bioinformation. 2017;13(11):356-9. Doi: 10.6026/97320630013356
Derardja I, Rebai R, Toumi ME, Kebaili FF, Boudah A. Identification of New Potential Cyclooxygenase-2 Inhibitors Using Structure-Based Virtual Screening, Molecular Dynamics and Pharmacokinetic Modelling. Biology and Life Sciences Forum [Internet]. 2024; 35(1).
Hano C, Tungmunnithum D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines (Basel). 2020;7(5). Doi: 10.3390/medicines7050026
Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, Alshabrmi FM, Palai S, Deb PK, Devi R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front Pharmacol. 2022;13:806470. Doi: 10.3389/fphar.2022.806470
Ahmadi M, Bekeschus S, Weltmann KD, von Woedtke T, Wende K. Non-steroidal anti-inflammatory drugs: recent advances in the use of synthetic COX-2 inhibitors. RSC Med Chem. 2022;13(5):471-96. Doi: 10.1039/d1md00280e
El-Malah AA, Gineinah MM, Deb PK, Khayyat AN, Bansal M, Venugopala KN, Aljahdali AS. Selective COX-2 Inhibitors: Road from Success to Controversy and the Quest for Repurposing. Pharmaceuticals (Basel). 2022;15(7). Doi: 10.3390/ph15070827
Patrono C. Cardiovascular effects of cyclooxygenase-2 inhibitors: a mechanistic and clinical perspective. Br J Clin Pharmacol. 2016;82(4):957-64. Doi: 10.1111/bcp.13048
Khan S, Andrews KL, Chin-Dusting JPF. Cyclo-Oxygenase (COX) Inhibitors and Cardiovascular Risk: Are Non-Steroidal Anti-Inflammatory Drugs Really Anti-Inflammatory? Int J Mol Sci. 2019;20(17). Doi: 10.3390/ijms20174262
Laloo D, Sinha SK, Prasad SK, Hemalatha S. Gastric H+, K+-ATPase inhibitory effects of the active constituent isolated from Potentilla fulgens roots: An in vivo and in silico molecular docking studies. Phytomed Plus. 2021;1(3):100037. Doi: 10.1016/j.phyplu.2021.100037
Muh'd Mb, Uzairu A, Shallangwa GA, Uba S. Molecular docking and quantitative structure-activity relationship study of anti-ulcer activity of quinazolinone derivatives. J King Saud Univ Sci. 2020;32(1):657-666. Doi: 10.1016/j.jksus.2018.10.003
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines (Basel). 2018;5(3). Doi: 10.3390/medicines5030093
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270-278. Doi: 10.4161/oxim.2.5.9498
Baier A, Szyszka R. Compounds from Natural Sources as Protein Kinase Inhibitors. Biomolecules. 2020;10(11). Doi: 10.3390/biom10111546
Bharate SB, Lindsley CW. Natural Products Driven Medicinal Chemistry. J Med Chem. 2024;67(23):20723-20730. Doi: 10.1021/acs.jmedchem.4c02736
Vennelakanti V, Qi HW, Mehmood R, Kulik HJ. When are two hydrogen bonds better than one? Accurate first-principles models explain the balance of hydrogen bond donors and acceptors found in proteins. Chem Sci. 2021;12(3):1147-1162. Doi: 10.1039/d0sc05084a
Derewenda ZS. C-H Groups as Donors in Hydrogen Bonds: A Historical Overview and Occurrence in Proteins and Nucleic Acids. Int J Mol Sci. 2023;24(17). Doi: 10.3390/ijms241713165
Pang X, Xu W, Liang J, Liu Y, Li H, Chen L. Research progress and perspectives of dual-target inhibitors. Eur J Med Chem. 2025;289:117453. Doi: 10.1016/j.ejmech.2025.117453
Yakkala PA, Kamal A. Dual-targeting inhibitors involving tubulin for the treatment of cancer. Bioorg Chem. 2025;156:108116. Doi: 10.1016/j.bioorg.2024.108116
Ju Z, Li M, Xu J, Howell DC, Li Z, Chen FE. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm Sin B. 2022;12(6):2790-2807.
Tajdari M, Peyrovinasab A, Bayanati M, Ismail Mahboubi Rabbani M, Abdolghaffari AH, Zarghi A. Dual COX-2/TNF-α Inhibitors as Promising Anti-inflammatory and Cancer Chemopreventive Agents: A Review. Iran J Pharm Res. 2024;23(1):e151312. Doi: 10.5812/ijpr-151312