Structure-Activity Relationship Among the Antibacterial Pterocarpans from African Erythrina Species: A Review
Main Article Content
Abstract
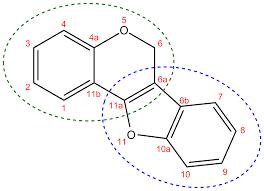
The genus Erythrina, a folkloric medicinal plant found in many tropical regions of the world, has yielded many flavonoids, including isoflavonoids of diverse structures. Some African Erythrina species have been investigated, and many have been found to contain isoflavonoids belonging to the sub-class pterocarpans which have displayed structural variations and interesting antibacterial activities. In this review, the structures and antibacterial activities of 14 pterocarpans reported in the literature from 8 African Erythrina species have been collated and subjected to structure-activity relationship (SAR) analysis to establish the best structural characteristics that enhance their antibacterial activities. The Structure-activity relationship (SAR) showed that their antibacterial activity against Gram-positive bacteria typified by Staphylococcus aureus, and the acid-fast organisms represented by Mycobacterium smegmatis, depends on the substitution pattern of the prenyl and hydroxyl groups on the two aromatic rings of the pterocarpan nucleus as well as the planarity of the pterocarpan molecule. Also, the high lipophilicity of the molecule provided by the presence of the prenyl groups enhances their antibacterial activity. Consequently, among the pterocarpans and indeed those from the African Erythrina species, 3,9-dihydroxypterocarpan (MIC=1.56 µg/mL), erycristagallin (MIC=3.13 µg/mL) and erythrabyssin II (MIC=3.12ug/mL) possess the best structural requirements for antibacterial activity against Staphylococcus aureus and Mycobacterium smegmatis. This review will guide the development of new antibacterial agents based on the pterocarpan framework.
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Nontokozo ZM and Mthokozisi BCS. Herbal Medicine. Builders, FP. (Ed.); Publishers: IntechOpen 2019. doi: 10.5772/intechopen.69412.
Chelliah S, Brian CJ, Sandhya P, Daniel M, Romasamy T. Pterocarpan scaffold: A natural lead molecule with diverse pharmacological properties. Eur. J. of Med. Chem. 2017; 128:219-236.
Aparna K, Munit S, Nuvneet, Munish S. Ethnomedicinal, phytochemical, therapeutical and pharmacological review of the genus Erythrina. Int .J. of Bot. Stud. 2020; 5(6):642-648.
Douglas FR, Blegelmeyer R, Toson NSB, Dreschi RR, Moreno PRH, Henriques AT. The genus Erythrina L. A review of its alkaloids, preclinical and clinical studies. Phytother Res. 2019; 33(5):1258-1276.
Nouran MF, Eman A, Mohamed E, Abdel NS. A comprehensive review on flavonoids biological activities of Erythrina plant species. J. of Ind. Crops Prod. 2018; 123: 500-538. https://doi.org/10.1016/j.indcrop.2018.06.028.
Soto-Hernandez R, Garda-Mateos R, Mguei-Chavez RS, Kite G, Martinez-Vazquez M and Ramos-Valdivia AC. Bioactive compounds in phytomedicine. In: Erythrina, a potential source of chemicals from the neotropics. Iraj Rascool (Ed). 2012; www.intechopen.com.
Anjum A, Sultan MZ, Ferdosh S, Islam MK, Rashid MA, Nahar L and Sarkar SD. Flavonoid, pterocarpans, and steroid from Erythrina fusca Lour growing in Bangladesh: isolation and antimicrobial and free-radical scavenging activity. J. Med. Plants 2021; 20 (79):37-46.
Rahmawati R, Hartai YW, Latip JB and Herlina T. An overview of techniques and strategies for isolation of flavonoids from the genus Erythrina. J. of Separt. Sci. 2023; 46(12). https://doi.org/10.1002/jssc.202200800.
Njamen D. Anti-inflammatory activity of erycristagallin, a pterocarpene from Erythrina mildbraedii. Eur. J. of Pharmacol. 2003; 468(1):67-74.
Machumi F, Bojase-Moleta G, Mapitse R, Masesane I, and Majinda RRT. Radical scavenging -flavonoids from Erythrina abyssinica. Nat. Prod. Comm. 2006; 1(4):287-292.
Inuma M, Tanaka T, Mizuno M, Yamamoto H, Kobayashi Y and Yonemori S. Phenolic compounds of Erythrina x bidwilli and their activity against oral microbial organisms. Chem. and Pharm. Bull. 1992; 40:2749-2752.
Dagne E, Gunatilaka AA and Kingston DGI.. Two bioactive pterocarpans from Erythrina bunana. J. of Nat. Prod.1993; 56:1831-1834.
Ingham JJ and Markham KR. Identification of the Erythrina phytoalexin, cristacarpin, and a note on the charility of other 6a-hydroxypterocarpans. Phytochem. 1980;19:1203-1209.
Mitscher LA, Gollapudi SR, Gerlach DC, Drake SD, Veliz EA and Ward JA.. Erycristin, a new antimicrobial from Erythrina crista-galli. Phytochem. 1988; 27:381-385.
Nkengfack AE, Vouffo TW, Vardamides JC, Kouam J, Fomum ZT, Meyer M, and Steiner O. Phenolic metabolites from Erythrina species. Phytochemistry. 1997;46:573-578.
Mitscher LA, Drake S, Gollapudi SR and Okwute SK. A modern look at folkloric use of anti-infective agents. J. of Nat. Prod. 1987;50:1025-1046.
Mitscher LA, Okwute SK.,Gollapudi SR, Drake S and Avona E. Antimicrobial pterocarpans of Nigerian Erythrina mildbraedii. Phytochem. 1988; 27:3449-3452.
Nkengfack AE, Vouffo TW, Fomum ZT, Meyer M, Bergendoff T and Steiner O. Prenylated isoflavanone from
the roots of Erythrina sigmoidea. Phytochemistry 1994; 38:1047-1051.
Deshpande VH, Pendse AD and Pendse R. Erythrinins A, B and C, three new isoflavones from the bark of Erythrina variegata. Ind. J. of Chem. 1977; 158:205-207.
Telikepalli H, Gollapudi SR, Kashavarz-Shokri A, Velazouez L, Sandman. RA, Veliz EA, Rao KVJ, Madhavi S, Mitscher LA. Isoflavonoids and a cinnamyl phenol from root extracts of Erythrina variegata. Phytochem. 1990; 29:2005-2007.
Wikipedia. en-wikipedia.org/wiki/structure-activity-relationship; retrieved ,21st July, 2024; last updated 7th April,2024.
Ahmad A, Elisha IL, van Vuuren S, and Viljoen A. Volatile phenolics: A comprehensive review of the anti-infective
properties of an important class of essential oil constituents. Phytochem. 2021; 90: 112864. doi.org/10.1016/j.phytochem2021.112864.
Mohamed AT and Amr E. Pederins, mycalamides, onnamides, and theopederins: Distinctive polyketide families with intriguing therapeutic potentialities. Curr. Res. in Biotechnol. 2023; 6:100145.
Rameshwar SC, Sachin DS, Jaya P, Ambhore SRC, Sanjay BB. Quinazoline: an update on the current status against convulsions. J.of Mol. Struct. 2022;1248:131384.
https:doi.org/10.1016/j.molstruc.2021.131384;
Lijuan W, Xiaonan Z, Jun Z, Menglin Y, Jinbo Y, Peiju Q. The therapeutic effects of marine sulfated polysaccharides on diabetic nephropathy. Int. J. of Biol. Macromol. 2024; 261, Part 1: 129269.https://doi.org/10.1016/j.ijibomac2024.129269.
Echeverria J, Opazo J, Mendoza L, Urzua A and Wilkens M. Structure-activity relationships of selected antibacterial natural flavones and flavanones of Chilean flora. Mol. 2017: 1-15.
Leticia JG, Carmen HC, Mariam AC, Manuel MD, and Igracio, RG. Synthesis of pterocarpans. Nat. Prod. Comm. 2011; 6(4):537-554.
Taylor TA, Unakal, CG. Staphylococcus aureus infection. In: StatPearls[Internet]. StatPearls Publishing 2024, Updated July 17, 2023; https;//ncbi.nlm.gov/books/NBK441868/.
Sparks IL, Derbyshire KM, Jacobs WR Jr, Morita YS. Mycobacterium smegmatis: The Vanguard of Mycobacterial Research. J Bacteriol. 2023;205(1):e0033722. doi: 10.1128/jb.00337-22. Epub.
Kyung-Min P, Seon JL, Hyungjong YYP, Ho-Sup J, Keesung K, Chang JL, and Pahn-Shick C. Hydrophilic and lipophilic characteristics of non-fatty acid moieties: significant factors affecting the antibacterial activity of lauric acid esters. 2018; 27(2):401-409. https://doi.org/10.1007/s/0068018-0353-x.
Akihisa M, Chikao N, Nobuyasu E, Shinkichi T. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochem. 1987; 26(8):2231-2234.
Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: Structure-
activity relationship and mechanism. Curr. Med Chem. 2015; 22(1):132-149.