Hematological Effects of Newbouldia laevis Leaf Extract and Homeopathic Remedies in Streptozotocin-Induced Diabetic Rats
Main Article Content
Abstract
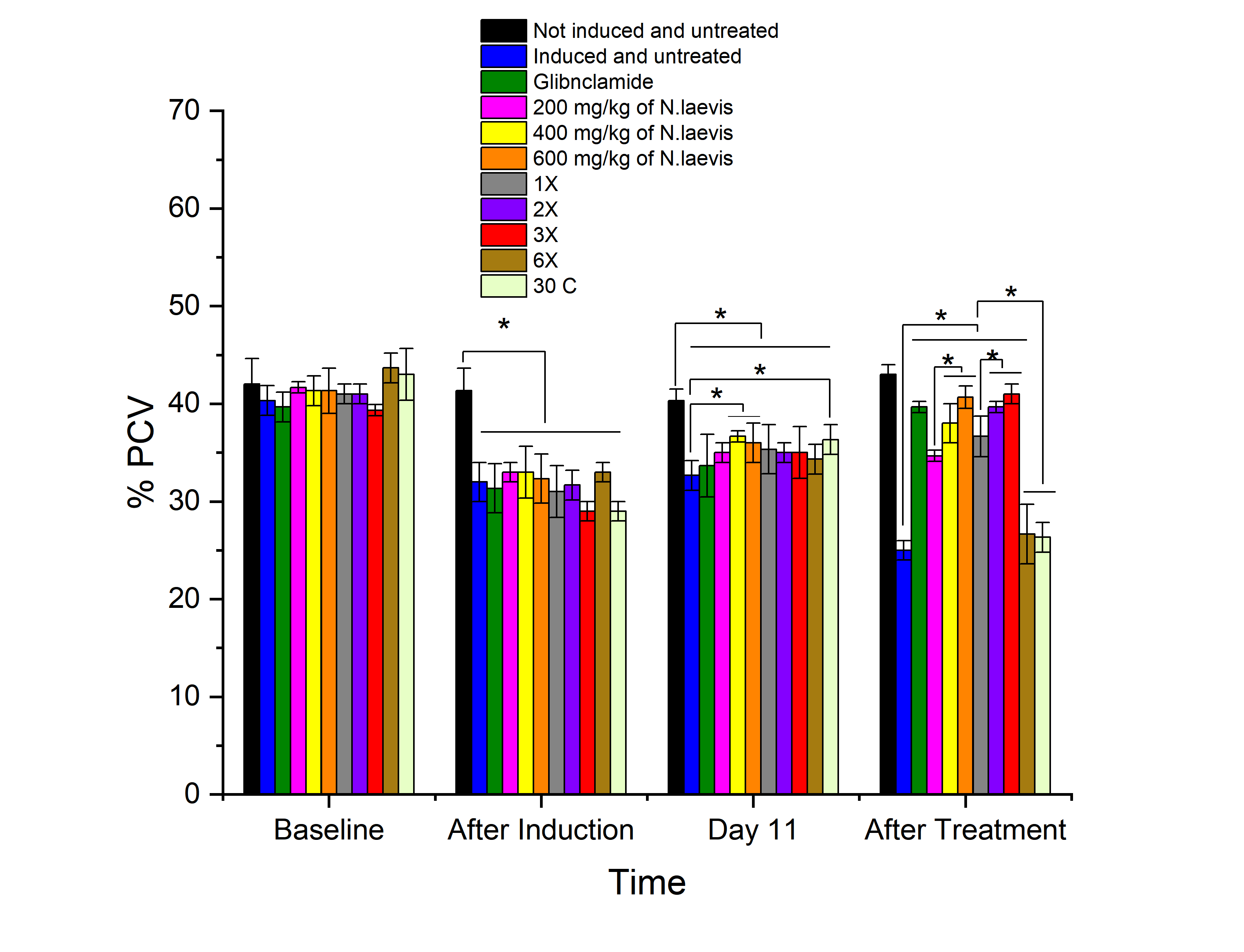
The defining characteristic of diabetes mellitus, a chronic metabolic disease, is persistent hyperglycemia caused by insufficient insulin secretion, defective insulin action, or both. This study evaluated the hematological effects of N. laevis leaf extract and its homeopathic formulations in diabetic rats induced with streptozotocin. The homeopathic mother tincture was prepared by dissolving the plant extract in absolute ethanol and further diluting it to obtain various X and C potencies via the serial dilution and succussion methods. Diabetes was induced in the rats via a single intraperitoneal injection of streptozotocin (50 mg/kg). The rats were assigned to eleven groups, including normal and diabetic controls; a standard drug group (glibenclamide); three crude extract doses (200, 400, and 600 mg/kg); and five homeopathic potencies (1X, 2X, 3X, 6X, and 30C). Treatments were administered orally for 21 days, and hematological parameters were determined via standard methods. The results showed that the 600 mg/kg dose and 3X potency of N. laevis significantly restored RBC, hemoglobin, and PCV levels and normalized WBC and platelet counts, comparable to those of the standard drug. In contrast, the 6X and 30C groups showed minimal improvements, with hematological parameters remaining similar to those of the diabetic control. These findings suggest that N. laevis possesses dose-dependent hematopoietic and immunomodulatory properties and may serve as a promising adjunctive treatment for diabetes-related hematological disorders.
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
References
Antar, S. A., Ashour, N. A., Sharaky, M., Khattab, M., Ashour, N. A., Zaid, R. T., Roh, E. J., Elkamhawy, A., and Al-Karmalawy, A. A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed Pharmacother.2023; 168:115734. https://doi.org/10.1016/j.biopha.2023.115734
Getawa, S., and Adane, T. Hematological abnormalities among adults with type 1 diabetes mellitus at the University of Gondar Comprehensive Specialized Hospital. SAGE Open Med.2022; 10: 20503121221094212. https://doi.org/10.1177/20503121221094212.
Galicia-Garcia, U., Benito-Vicente, A., Jebari, S., Larrea-Sebal, A., Siddiqi, H., Uribe, K. B., Ostolaza, H., and Martín, C. (2020). Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 21(17):6275. https://doi.org/10.3390/ijms21176275.
Eluu, S. C., Oko, A. O., Eluu, K., Okoye, C. S., Onyekwere, U. U., and Omoniyi, O. A. Impact of N. laevis root extract on hematological parameters in rats: A comprehensive study on dosage-dependent effects and long-term dynamics. Niger Agric J. 2023; 54(2):324–329.
Szablewski, L., and Sulima, A. (2017). The structural and functional changes of blood cells and molecular components in diabetes mellitus. Biol Chem. 2017;398(4):411–423.
https://doi.org/10.1515/hsz-2016-0254.
Ramachandran, A., and Snehalatha, C. Current scenario of diabetes in India. J Diabetes. 2009; 1(1):18–28.
Macciò, A., and Madeddu, C. Management of anemia of inflammation in elderly individuals. Anemia.2012; 563251. https://doi.org/10.1155/2012/563251
Eluu, S. C., Oko, A. O., Eluu, K., Onyekwere, U. U., and Okoye, C. S. Impact of N. laevis root extract on liver enzymes in rats. Niger Agric J, 2023: 54(2): 259–264.
Mbagwu, I. S., Akah, P. A., andAjaghaku, D. L. (2020). Newbouldia laevis improved glucose and fat homeostasis in a type-2 diabesity mice model. J. of Ethnopharm.2020.251: 112555.
Rawat, P. S. Select your Dose and Potency 2016., B. Jain Publishers (P) Ltd. Pg 17
Tella, T., Masola, B., and Mukaratirwa, S. (2022). Anti-diabetic potential of Psidium guajava leaf in streptozotocin-induced diabetic rats. Phytomed. Plus, 2(2), 100254.
Ochei, J., and Kolhatkar, A. (2008). Medical laboratory science: Theory and practices (pp. 311–347). Tata McGraw-Hill.
Baker, F S and Silverton, R. E. Introduction to Medical Laboratory Technology, 1982; 5thedition, Butterworth S. C., London, Pp. 481 – 494.
Rehman, K., and Akash, M. S. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked? J Cell Biochem.2017; 118:3577–3585.
Decourt B, D'Souza GX, Shi J, Ritter A, Suazo J, Sabbagh MN. The cause of Alzheimer’s disease: The theory of multipathology convergence to chronic neuronal stress. Aging Dis. 2022; 13:37–60.
Azeez, O. I., Oyagbemi, A. A., Oyeyemi, M. O., andOdetola, A. A. (2010). Ameliorative effects of Cnidoscolus aconitifolius on alloxan toxicity in Wistar rats. Afr Health Sci.2010; 10(3): 283–291.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3035966/
Osigwe, C. C., Akah, P. A., Nworu, C. S., Okoye, T. C., andTchimene, M. K. Antihyperglycemic studies on the leaf extract and active fractions of N. laevis (Bignoniaceae). Pharmacol Pharm.2015: 6(11).
Ravanan, R., Spiro, J. R., Mathieson, P. W., and Smith, R. M. Impact of diabetes on hemoglobin levels in renal disease. Diabetologia.2017; 50(1), 26–31.
Rossing, K., Christensen, P. K., Hovind, P., Tarnow, L., Rossing, P., and Parving, H. H. Progression of nephropathy in type 2 diabetic patients. Kidney Int.2004; 66: 1596–1605. https://doi.org/10.1111/j.1523-1755.2004.00925.x
Ranil, P. K., Raman, R., Rachepalli, S. R., Pal, S. S., Kulothungan, V., Lakshmipathy, P., Satagopan, U., Kumaramanickavel, G., and Sharma, T. Anemia and diabetic retinopathy in type 2 diabetes mellitus. J Assoc Physicians India. 2010; 58:91–94.
Muhammad, N. O., and Oloyede, O. B. Hematological parameters of broiler chicks fed Aspergillus niger-fermented Terminalia catappa seed meal-based diet. Glob J BiotechnolBiochem.2009; 4:179–183.
Giacco, F., and Brownlee, M. Oxidative stress and diabetic complications. Circ Res, 2010; 107(9): 1058–1070. https://doi.org/10.1161/CIRCRESAHA.110.223545.
Etim, N. N., Williams, M. E., Akpabio, U., and Offiong, E. E. Hematological parameters and factors affecting their values. Agric Sci. 2014;2(1): 37–47.
Pickup, J. C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care.2004; 27(3): 813–823.
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. EurCardiol.2019;14(1):50-59. doi: 10.15420/ecr.2018.33.1. PMID: 31131037; PMCID: PMC6523054.
Demirtas, L., Degirmenci, H., Akbas, E. M., Ozcicek, A., Timuroglu, A., Gurel, A., and Ozcicek, F. Association of hematological indices with diabetes, impaired glucose regulation and microvascular complications of diabetes. Int J Clin Exp Med.2015; 8(7):11420–11427.
Vazzana, N., Ranalli, P., Cuccurullo, C., andDavì, G. (2012). Diabetes mellitus and thrombosis. Thromb. Res. 2012; 129(3):371–377.
Ayoola, A. A., Yusuf, A. O., and Oki, D. G. Phytochemical screening and proximate analysis of N. laevis and Allium sativum. Niger J Anim Sci, 2016; 1:242–256.